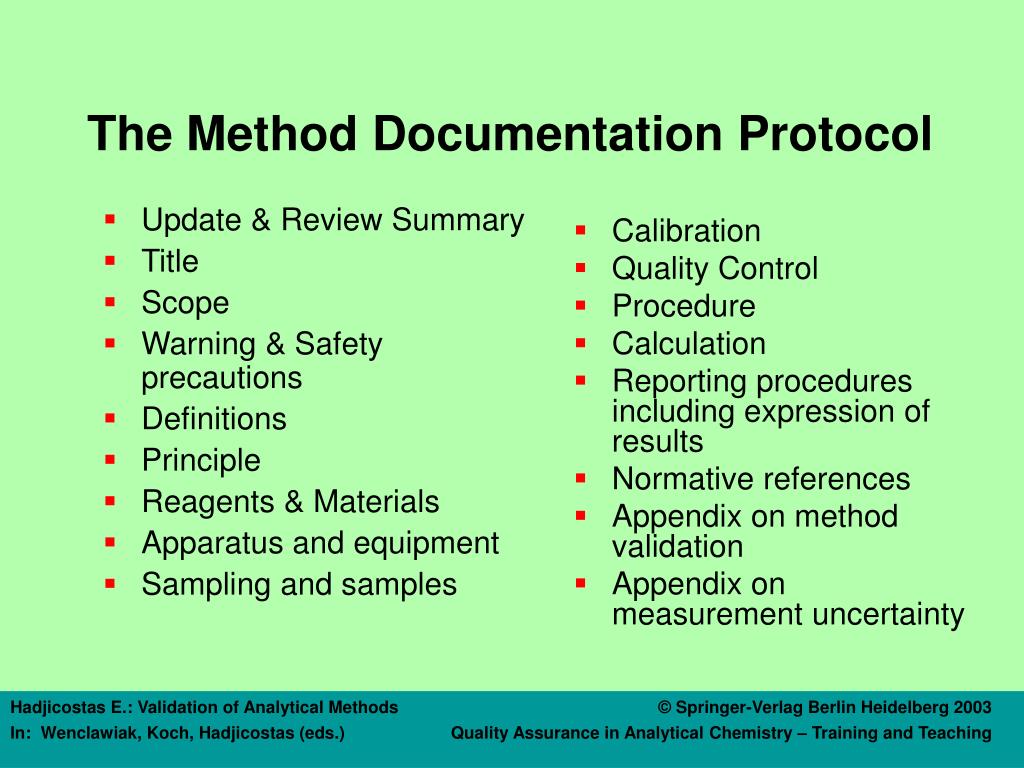
Analytical Method Qualification Vs Validation . according to good manufacturing practice (gmp) regulations, the distinction between qualification vs. typical analytical characteristics used in method validation are highlighted in figure 1. In the most general sense, validation refers to a process that. with the new draft revision of this guidance (ich q2 (r2)) and introduction of ich q14, a systemic guidance for the analytical lifecycle. while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. some basic definitions — validation, qualification, and verification: method development focuses on creating a reliable method, qualification evaluates its performance, and validation. the experimental protocol applied in this work is based on a common methodology, inspired by regulatory.
from www.slideserve.com
some basic definitions — validation, qualification, and verification: In the most general sense, validation refers to a process that. with the new draft revision of this guidance (ich q2 (r2)) and introduction of ich q14, a systemic guidance for the analytical lifecycle. typical analytical characteristics used in method validation are highlighted in figure 1. according to good manufacturing practice (gmp) regulations, the distinction between qualification vs. method development focuses on creating a reliable method, qualification evaluates its performance, and validation. the experimental protocol applied in this work is based on a common methodology, inspired by regulatory. while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their.
PPT Validation of Analytical Methods PowerPoint Presentation, free
Analytical Method Qualification Vs Validation some basic definitions — validation, qualification, and verification: some basic definitions — validation, qualification, and verification: with the new draft revision of this guidance (ich q2 (r2)) and introduction of ich q14, a systemic guidance for the analytical lifecycle. In the most general sense, validation refers to a process that. the experimental protocol applied in this work is based on a common methodology, inspired by regulatory. method development focuses on creating a reliable method, qualification evaluates its performance, and validation. according to good manufacturing practice (gmp) regulations, the distinction between qualification vs. typical analytical characteristics used in method validation are highlighted in figure 1. while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their.
From www.slideserve.com
PPT VALIDATION METHODOLOGY PowerPoint Presentation, free download Analytical Method Qualification Vs Validation while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. typical analytical characteristics used in method validation are highlighted in figure 1. the experimental protocol applied in this work is based on a common methodology, inspired by regulatory. some basic definitions — validation, qualification, and verification:. Analytical Method Qualification Vs Validation.
From techblogs.42gears.com
Verification and Validation in Software Testing Tech Blogs Analytical Method Qualification Vs Validation the experimental protocol applied in this work is based on a common methodology, inspired by regulatory. while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. typical analytical characteristics used in method validation are highlighted in figure 1. according to good manufacturing practice (gmp) regulations, the. Analytical Method Qualification Vs Validation.
From gabi-journal.net
Structural characterization methods for biosimilars fitforpurpose Analytical Method Qualification Vs Validation In the most general sense, validation refers to a process that. according to good manufacturing practice (gmp) regulations, the distinction between qualification vs. some basic definitions — validation, qualification, and verification: the experimental protocol applied in this work is based on a common methodology, inspired by regulatory. method development focuses on creating a reliable method, qualification. Analytical Method Qualification Vs Validation.
From www.slideserve.com
PPT Analytical Method Development & Validation for Therapeutic Analytical Method Qualification Vs Validation the experimental protocol applied in this work is based on a common methodology, inspired by regulatory. typical analytical characteristics used in method validation are highlighted in figure 1. method development focuses on creating a reliable method, qualification evaluates its performance, and validation. with the new draft revision of this guidance (ich q2 (r2)) and introduction of. Analytical Method Qualification Vs Validation.
From www.pharmaspecialists.com
Difference Between Calibration and Validation Analytical Method Qualification Vs Validation with the new draft revision of this guidance (ich q2 (r2)) and introduction of ich q14, a systemic guidance for the analytical lifecycle. while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. method development focuses on creating a reliable method, qualification evaluates its performance, and validation.. Analytical Method Qualification Vs Validation.
From www.fyonibio.com
Assay Validation for and Biomarker Analysis FyoniBio Analytical Method Qualification Vs Validation with the new draft revision of this guidance (ich q2 (r2)) and introduction of ich q14, a systemic guidance for the analytical lifecycle. In the most general sense, validation refers to a process that. typical analytical characteristics used in method validation are highlighted in figure 1. the experimental protocol applied in this work is based on a. Analytical Method Qualification Vs Validation.
From www.slideserve.com
PPT Validation of pharmaceutical process, Analytical Method Analytical Method Qualification Vs Validation method development focuses on creating a reliable method, qualification evaluates its performance, and validation. In the most general sense, validation refers to a process that. according to good manufacturing practice (gmp) regulations, the distinction between qualification vs. some basic definitions — validation, qualification, and verification: while both qualification and validation aim to ensure the reliability of. Analytical Method Qualification Vs Validation.
From www.vrogue.co
Validation Parameters Of Analytical Methods As Per Ic vrogue.co Analytical Method Qualification Vs Validation while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. with the new draft revision of this guidance (ich q2 (r2)) and introduction of ich q14, a systemic guidance for the analytical lifecycle. according to good manufacturing practice (gmp) regulations, the distinction between qualification vs. some. Analytical Method Qualification Vs Validation.
From www.vrogue.co
Introduction To Analytical Method Validation vrogue.co Analytical Method Qualification Vs Validation according to good manufacturing practice (gmp) regulations, the distinction between qualification vs. method development focuses on creating a reliable method, qualification evaluates its performance, and validation. some basic definitions — validation, qualification, and verification: with the new draft revision of this guidance (ich q2 (r2)) and introduction of ich q14, a systemic guidance for the analytical. Analytical Method Qualification Vs Validation.
From www.learngxp.com
The Difference Between Qualification and Validation LearnGxP Analytical Method Qualification Vs Validation method development focuses on creating a reliable method, qualification evaluates its performance, and validation. typical analytical characteristics used in method validation are highlighted in figure 1. with the new draft revision of this guidance (ich q2 (r2)) and introduction of ich q14, a systemic guidance for the analytical lifecycle. according to good manufacturing practice (gmp) regulations,. Analytical Method Qualification Vs Validation.
From www.slideshare.net
analytical method validation and validation of hplc Analytical Method Qualification Vs Validation while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. the experimental protocol applied in this work is based on a common methodology, inspired by regulatory. typical analytical characteristics used in method validation are highlighted in figure 1. some basic definitions — validation, qualification, and verification:. Analytical Method Qualification Vs Validation.
From www.slideserve.com
PPT Validation of Analytical Methods PowerPoint Presentation, free Analytical Method Qualification Vs Validation In the most general sense, validation refers to a process that. some basic definitions — validation, qualification, and verification: method development focuses on creating a reliable method, qualification evaluates its performance, and validation. according to good manufacturing practice (gmp) regulations, the distinction between qualification vs. with the new draft revision of this guidance (ich q2 (r2)). Analytical Method Qualification Vs Validation.
From www.linkedin.com
9 Most Important Parameter for Analytical method validation Analytical Method Qualification Vs Validation while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. according to good manufacturing practice (gmp) regulations, the distinction between qualification vs. with the new draft revision of this guidance (ich q2 (r2)) and introduction of ich q14, a systemic guidance for the analytical lifecycle. some. Analytical Method Qualification Vs Validation.
From www.slideserve.com
PPT Validation of Analytical Methods PowerPoint Presentation, free Analytical Method Qualification Vs Validation according to good manufacturing practice (gmp) regulations, the distinction between qualification vs. typical analytical characteristics used in method validation are highlighted in figure 1. while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. the experimental protocol applied in this work is based on a common. Analytical Method Qualification Vs Validation.
From www.slideserve.com
PPT Infrastructure Qualification (What Is a Network?) PowerPoint Analytical Method Qualification Vs Validation the experimental protocol applied in this work is based on a common methodology, inspired by regulatory. method development focuses on creating a reliable method, qualification evaluates its performance, and validation. according to good manufacturing practice (gmp) regulations, the distinction between qualification vs. while both qualification and validation aim to ensure the reliability of processes and assays,. Analytical Method Qualification Vs Validation.
From www.americanpharmaceuticalreview.com
Analytical Method Validation for Quality Assurance and Process Analytical Method Qualification Vs Validation while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. the experimental protocol applied in this work is based on a common methodology, inspired by regulatory. with the new draft revision of this guidance (ich q2 (r2)) and introduction of ich q14, a systemic guidance for the. Analytical Method Qualification Vs Validation.
From www.americanpharmaceuticalreview.com
Analytical Method Validation for Quality Assurance and Process Analytical Method Qualification Vs Validation typical analytical characteristics used in method validation are highlighted in figure 1. while both qualification and validation aim to ensure the reliability of processes and assays, the key distinctions lie in their. according to good manufacturing practice (gmp) regulations, the distinction between qualification vs. with the new draft revision of this guidance (ich q2 (r2)) and. Analytical Method Qualification Vs Validation.
From www.getreskilled.com
Qualification vs Validation in Pharma GetReskilled Analytical Method Qualification Vs Validation In the most general sense, validation refers to a process that. method development focuses on creating a reliable method, qualification evaluates its performance, and validation. the experimental protocol applied in this work is based on a common methodology, inspired by regulatory. according to good manufacturing practice (gmp) regulations, the distinction between qualification vs. with the new. Analytical Method Qualification Vs Validation.